107. Prevention and
management of eclampsia.
The management of pre-eclampsia should begin at the first
sign
of abnormality, well before the diagnosis is confirmed. When
excessive weight gain or fluid retention is documented in the absence of other
pathognomonic changes, a brief dietary history should be
obtained to look for indiscretions and excesses. Appropriate
counseling should follow. The patient should be advised of
any concerns
and be requested to increase periods of bed rest, preferably
in
the left lateral position. For the following 48 hours,
activity out of bed
should be limited to eating meals (not preparing them) and
using the
bathroom. A no-added-salt diet may be prescribed. More
Severe
sodium restriction is contraindicated for all but those in
frank renal
failure. Follow-up is requested 48 hours or less later to
ton-firm
continued normal blood pressure and to determine the
efficacy of
treatment for the weight gain and fluid retention.
Successful treatment
dictates no further intervention, other than perhaps the
continuation of
the no-added-salt diet. If there has been no weight loss,
continued
reduction of activity with increased periods of bed rest and
more
frequent prenatal visits are indicated.
The treatment of hypertension depends to a great extent on
the
duration of the pregnancy and the elevation of blood
pressure. At the
lowest end of the hypertensive spectrum, 140/90 mm Hg, and
in
the absence of proteinuria, outpatient management is
possible.
Mild salt reduction (no added salt) and bed rest in the left
lateral
position are again advised. The patient and her family
should be
counseled regarding warning symptoms of deterioration.
Follow-up
should occur no later than 48 hours. Many patients in this
category
respond to bed rest with a normalization of blood pressure.
These
women merely require more frequent follow-up than usual.
For the nonresponders, the next step should be a trial of
bed rest
and a no-added-salt diet in the more controlled environment
of the
hospital. If blood pressure normalizes, observation should
be
continued for an additional 24 to 48 hours and the patient
should
follow a regimen of continued bed rest and diet with
frequent followup.
Nonresponders who are 37 weeks' gestation or greater should
be
evaluated for induction of labor. Those at less than 37
weeks' gestation
should continue bed rest and diet in the hospital for
several days while
undergoing the work-up detailed in Table. Patients with
continued
mild hypertension (not greater than 150/100 mm Hg) without
proteinuria and with normal laboratory values may be
considered for discharge and close follow-up.
108. Management of
nephropathy.
Pregnant patients with kidney disease are often under the
care of a maternal-fetal specialist who has advanced training in high-risk
obstetrics. These patients receive frequent obstetric follow-up that includes
careful blood pressure monitoring, renal function testing, and 24-hour urine
protein collections. Consultation with a nephrologist often occurs, particularly
for patients with more advanced disease and those with progressive renal
failure.
Almost all patients with significant renal disease and/or
hypertension in late pregnancy, or when the likelihood of fetal viability is
very high, are delivered and they can be managed as non-gravid patients. If
progressive renal failure occurs either in early pregnancy or before fetal
viability can be assured, however, dialysis may need to be considered.
Dialysis should be initiated when the serum creatinine level
is 3.5-5.0 mg/dL or the glomerular filtration rate (GFR) is below 20 ml/min.
Fetal outcome is improved with longer, more frequent hemodialysis sessions,
which usually involves 20 hours of dialysis per week. Daily dialysis is more
likely to prevent hypotension and significant metabolic shifts.
Dialysis should aim to keep blood urea nitrogen levels below
50 mg/dL, because controlling uremia may avoid polyhydramnios, control
hypertension, and improve the mother's nutritional status. Peritoneal dialysis
with smaller volumes and frequent exchanges can also be done to achieve these
same goals.
Anemia should be treated with erythropoietin and careful attention to iron therapy. Nutritional support that allows weight gains
of 0.3 to 0.5 kg/wk should be maintained in the second and third trimesters.
Pregnancy in patients
receiving dialysis
Although fertility is significantly impaired in women with
end-stage renal disease, pregnancy may still occur.[14] Most
women on dialysis are anovulatory, with either irregular or no menses, which
can result in significant delays in the diagnosis of pregnancy in those who do
conceive. In addition, the spontaneous abortion rate for pregnant women who
require dialysis is approximately 50%. For pregnancies that continue, however,
the fetal survival rate is as high as 71%.
Pregnancy in women
with kidney transplants
Pregnancy in women following renal transplantation has
become commonplace. Transplantation restores fertility, and although most women
with kidney transplants can deliver successfully, there is a higher risk of
miscarriage, therapeutic abortion, stillbirth, ectopic pregnancy, preterm
birth, low birthweight babies, and neonatal death.
Guidelines for pregnancy in kidney transplant recipients
include the following
·
Good general health for 2 years
post-transplantation, with serum creatinine levels below 2.0 mg/dL (preferably
< 1.5 mg/dL)
·
No recent acute rejection or ongoing rejection
·
Normotension, or hypertension
controlled with minimal antihypertensive agents
·
No or minimal proteinuria
·
No evidence of pelvicaliceal dilatation
on renal ultrasonogram
Recommended immunosuppression in kidney transplant
recipients includes:
·
Calcineurin inhibitor–based therapy at
appropriate therapeutic levels
·
Breast-feeding on cyclosporine is not recommended; tacrolimus may be taken during breast-feeding though monitoring
of infant levels is recommended.
·
If necessary, methylprednisolone is the preferred agent for treatment of rejection
should it occur during pregnancy
The following are complication risks in kidney transplant
recipients:
·
Immunosuppressive agents increase the
risk of hypertension during pregnancy
·
Preeclampsia occurs in approximately
one third of kidney-transplant recipients
·
Almost 50% of pregnancies in these
women end in preterm delivery due to hypertension
·
Blood levels of calcineurin inhibitors
need to be frequently monitored due to changes in volumes of distribution of
extracellular volume
·
There is an increased risk of
cytomegalovirus, toxoplasmosis, and herpes infections, which raise concern for
the fetus
109. Transverse and
oblique fetal positions. Course and outcomes of labor.
Note: Guys I added a bit from malpresentation, I
couldn’t find everything
With transverse and oblique lies, the shoulder structures
(acromion) can be used for the description of position. The incidence of
transverse lie has been reported as 0.3%.2 Fetal malformations can cause these
(and other) malpresentations. These two types of lies have been associated with
grandparity, contracted maternal pelvis, placenta previa, prematurity,
polyhydramnios, and uterine abnormalities such as transverse septum or
leiomyomas. A patient in her last month of pregnancy with a fetus in a
transverse or oblique lie would require an ultrasound and physical examination
for the evaluation of any of the above risk factors. If no contraindications
are identified, one possible management alternative would be an external
cephalic version. The success of external cephalic version in cases of
transverse lie has been reported as high as 83%,3 even when performed
intrapartum. If an elective cesarean section is to be performed, it should be
scheduled for the last week of the pregnancy, because 83% of transverse lies
will spontaneously convert to a vertex presentation before 39 weeks.4 This
expectant management has to be individualized. Patients showing signs of early
labor or those with cervical dilation are candidates for an attempt of external
version or delivery by cesarean section before 39 weeks.
Vital
to good progress in labour is the tight application of the fetal presenting
part on to the cervix. Face presentations may apply themselves poorly to the
cervix and the resulting progress in labour may be poor, although vaginal birth
is still possible. Brow presentations are associated with the mento-vertical
diameter, which is simply too large to fi t through the bony pelvis unless fl
exion occurs or hyperextension to a face presentation. Brow presentation
therefore often manifests as poor progress in fi rst stage, often in a multiparous
woman. Shoulder presentations cannot deliver vaginally and once again poor
progress will occur. Malpresentations are more common in women of high parity
and some carry a risk of uterine rupture
if
the labour is allowed to continue.
110. Obstetric
analgesia. Use of medications, electroanalgesia, acupuncture.
Analgesic and Anesthetic Techniques for
Obstetrics
Labor and
Delivery
Nonpharmacologic
Lamaze
Leboyer
Acupuncture
Hypnosis
Massage
Warm baths
Regional
Analgesia/Anesthesia
Local infiltration for episiotomy (lidocaine)
Epidural (bupivacaine)
Spinal (tetracaine, bupivacaine, or
lidocaine)
Caudal
Paracervical
Pudendal (lidocaine)
Systemic Medication
Narcotics (fentanyl)
Sedativ-tranquilizers (Phenergan)
Inhalation
Analgesia
Nitrous oxide
Penthran
Ethran
Cesarean Section
Regional
Anesthesia
Epidural (lidoraine, chloroprocaine, or
bupivacaine)
Spinal (tetracaine, bupivacaine, or
lidocaine)
Pudendal nerve block
General Anesthesia
Although all drugs cross the placenta to some
extent, the degree of
transfer is determined by maternal and fetal
blood flow to the placenta as well as by factors that determine drug passage
across the placental barrier itself.
These inhalation anesthetics produce a
dose-dependent decrease in
uterine resting tone, uterine contractility,
and uterine responsiveness to oxytocin.
During the active phase of the first stage of
labor, epidural analgesia
has either no significant effect or causes
enhanced uterine activity.
Excessive anesthesia during the first stage of
labor relaxes the pelvic
floor musculature and may cause mal-rotation
by interfering with
internal rotation of the fetal head. In the
parturient who is motivated to bear down and is properly instructed, the
appropriate use of regional anesthesia without excessive motor blockade should
not prolong the second stage of labor.
Electroanalgesia
Transcutaneous electrical nerve stimulation TENS uses the
gate theory of pain control and, by application of an
electrical current to the nerves carrying the painful
stimuli, transmission of pain is partially blocked. Skin surface
electrodes (Fig. 1) are used to apply a low voltage
electrical current, which is modified by the patient. These are usually applied
across the lower back covering the T10-L1 nerve roots (theinnervation of the
uterus) early in the first stage for optimum effect. Although concern has been
expressed
about the use of TENS applied over the lower abdomen as the
electrical activity may theoretically have an effect
on the fetal heart, no adverse effect has been documented.
111. Clinic and
management of placental abruption.
Clinic
Placental abruption typically presents as vaginal bleeding
associated with pain. The pain can be constant, or as frequent short-lasting
contractions caused by the irritable effect of blood within
the uterus. The patient may report reduced fetal movements and
the cardiotocograph may demonstrate a non-reassuring fetal heart rate pattern.
Constant pain associated with a uterus that is very
hard on palpation is known as a Couvelaire uterus and is due to
a large volume of blood within the myometrium.
Management of placental
abruption
The patient should be initially resuscitated using the structured
approach of ABC. Management depends on recognition of the problem, realization
that true blood loss may be far greater than the
blood loss seen, and rapid institution of major haemorrhage
management. In very severe cases, the fetus will be dead and vaginal delivery
can be accelerated by artificial rupture of the membranes once the mother is
reasonably stable. If the fetus is alive, delivery without compromising the
mother’s resuscitation is urgent and this will usually be by Caesarean section.
112. Prevention and
management of uterus’ ruptures.
Prevention
113. Decapitation
(indications, conditions, technique).
DECAPITATION
Definition
It is a destructive
operation where by the fetal head is severed from the trunk and the delivery is
completed with the extraction of the trunk and that of the decapitated head per
vaginam.
Indication
Neglected shoulder
presentation with dead fetus where neck is easily assessable
Interlocking head of the
twins
Procedure
The operation should be
done at general anesthesia
Actual step
Step 1 :- if the fetal hand
is not prolapsed bring down the hand. A roller gauze is tied on the fetal wrist
and assistant is asked to give the traction towards the side away from the
fetal head to make the neck more assessable and fixed
Step 2 :- two fingers of the
left hand are introduced with the palmar surface downwards and the finger tips
are to be placed on the superior surface of the neck –the prolapsed site of
decapitation.
Step 3 :- the decapitation
hook with knife is to be introduced flushed under the guidance of the fingers
placed in to the vagina, trhe knob pointing toward the fetal head. The hook is
pushed above the neck and rotated to 90 ̊ , so as to placed the knife firmly
against the neck.
Step 4 :- by upward and
downward movement of the hook with knife the vertebral column is severed.
Step 5:- delivery of the
decapitation head – the methods are
- By
hooking the index fingers into the mouth
- By
holding the severed head with Giant Vulsellum and delivery of he head as
that of aftercoming head in breech.
- Using
forceps
Step 6 :- routine
exploration of the utero vaginal canal to exclude rupture of the uterus or any
other injury.
114. Flat pelvis.
Mechanisms of labor.
Normal
mechanism of labour
As the fetus descends through the birth canal, it undergoes changes
in position and attitude, in order to pass through the pelvis. In a typical
(gynaecoid) pelvis, the transverse diameter (13 cm) is wider than the
anteroposterior diameter (11 cm) at the pelvic inlet. The mid-cavity is
circular in cross-section (12 cm diameter). At the pelvic outlet the AP
diameter (13.5 cm) iswider than the transverse (11 cm).
ENGAGEMENT The fetal head enters the pelvis in the OT
position. The head is engaged when the widest part of the presenting part has
passed through the inlet (<2/5 palpable abdominally). This occurs prior to labour in nulliparous
women (although not necessarily in multiparous women).
DESCENT Descent occurs with uterine contractions.
FLEXION As the head enters the midcavity, it flexes
(chin touching chest), remaining in the OT position.
INTERNAL ROTATION As the head reaches the pelvic
outlet it encounters the levator ani muscles of the pelvic floor and rotates to
the OA position.
EXTENSION The head descends beyond the ischial spines.
Upward pressure from the pelvic floor causes the head to extend.
CROWNING The occiput emerges from underneath the
symphysis pubis and distends the vulva.
EXTERNAL ROTATION The shoulders now rotate into
an oblique or frank anterior/posterior plane.
RESTITUTION The head now aligns itself with the shoulders
and delivery occurs.
115. Management of the
second stage of labor.
Clinical Management of the Second Stage
As in the first stage, certain steps should be taken in the
clinical
management of the second stage of labor.
Maternal Position. With the
exception of avoiding the supine
position, the mother may assume any comfortable position for
effective bearing down. If the birth is to occur in another
room,
primiparous patients should be moved at or before the beginning
of crowning. Multiparous patients should be brought to the delivery room at the
time of complete cervical dilation.
Bearing Down. With each
contraction, the mother should be
encouraged to hold her breath and bear down with expulsive
efforts. This is particularly important for patients with regional anesthesia because
their reflex sensations may be impaired.
Fetal Monitoring. During the
second stage, the fetal heart rate
should be monitored continuously or evaluated every 5 minutes in
patients with obstetric risk factors. Fetal heart rate
decelerations (head compression or cord compression) with recovery following
the uterine contraction may normally occur during this stage.
Vaginal Examination. Progress
should be recorded
approximately every 30 minutes during the second stage.
Particular attention should be paid to the descent and flexion of the
presenting part, the extent of internal rotation, and the development of
molding or caput.
116. Diagnostic of
early terms of pregnancy.
The diagnosis of pregnancy requires a multifaceted approach
using 3 main diagnostic tools. These are history and physical examination,
laboratory evaluation, and ultrasonography.
History: The woman
should describe her usual menstrual pattern, including date of onset of last
menses, duration, flow, and frequency. Items that may confuse the diagnosis of
early pregnancy are an atypical last menstrual period, contraceptive use, and a
history of irregular menses.
physical examination: Upon
physical examination, one may find an enlarged uterus after bimanual
examination, breast changes, and softening and enlargement of the cervix (Hegar
sign; observed at approximately 6 wk). The Chadwick sign is a bluish
discoloration of the cervix from venous congestion and can be observed by 8-10
weeks. A gravid uterus may be palpable low in the abdomen if the pregnancy has
progressed far enough, usually by 12 weeks.
laboratory evaluation: assessment
of human chorionic gonadotropin (hCG) in urine or blood.
ultrasonography
117. Puerperium. Course
and management.
Puerperium
The puerperium consists of the period following delivery of the
baby and placenta to approximately 6 weeks postpartum. During
the puerperium, the reproductive organs and maternal physiology return toward
the prepregnancy state although menses may not return for much longer.
Anatomic and Physiologic Changes
Involution of the Uterus
Through a process of tissue catabolism, the uterus rapidly
decreases in weight from about 1000 g at delivery to 100 to 200
g at approximately 3 weeks postpartum. The cervix similarly loses its
elasticity and regains its prepregnancy firmness. For the first
few days after delivery, the uterine discharge (lochia) appears red (lochia rubra) owing to the
presence of erythrocytes. After 3 to 4 days, the lochia becomes paler (lochia serosal, and by the tenth
day, it assumes a white or yellow-white color (lochia alba). Foul-smelling
lochia suggests, but is not diagnostic of, endometritis.
Vagina
Although the vagina may never return to its prepregnancy state,
the supportive tissues of the pelvic floor gradually regain
their former tone.
Cardiovascular System
Immediately following delivery, there is a marked increase in
peripheral vascular resistance due to the removal of the
low-pressure uteroplacental circulatory shunt. The cardiac output and plasma
volume gradually return to normal during the first 2 weeks of the puerperium.
As a result of the loss of plasma volume and the diuresis of extracellular
fluid, a marked weight loss occurs in the first week. A significant granulocytic
leukocytosis may be seen in the immediate
postpartum period.
Psychosocial Changes
It is fairly common for women to exhibit a mild degree of
depression a few days following delivery. The "postpartum blues" are probably due to both emotional and hormonal factors. With
understanding and reassurance from both family and physician, this usually
resolves without consequence.
Return of Menstruation and Ovulation
In women who do not nurse, menstrual flow will usually return
by 6 to 8 weeks following delivery, although this is highly
variable.
Although ovulation may not occur for several months,
particularly in nursing mothers, contraceptive counseling and use should be
emphasized during the puerperium to avoid an undesired
pregnancy.
Management
Management of normal puerperium
Immediately following delivery, the patient should be
closely observed. She may be given
a drink of her choice or something to eat, if she is hungry.
Principles -
- To give all out attention in to restore the health status
of the mother.
- To prevent infection.
- To take care of the breasts, including promotion of
lactation and nursing of the child.
- To motivate the mother for contraception.
General management -
Rest and ambulance -
It is indeed difficult to categories an uniform period of
rest. After a good resting period,
the patient becomes fresh and can breast feed the baby or
moves out of bed to go to the
toilet. Early ambulation is encouraged.
Que. - What is the benefits of early ambulation after
delivery ?
Ans. - Advantages of
early ambulation are:
- Provide a
sense of well-being.
- Bladder
complications and constipation are reduced.
-
Facilitates uterine drainage.
- Hastens
involution of uterus.
- Lessens
puerperal venous thrombosis and embolism.
Hospital stay -
Early discharge from the hospital is an almost universal
procedure. If adequate supervision
by trained health visitors is provided, there is no harm in
early discharge.
Diet -
The patient should be on normal diet of her choice. If the
patient is lactating, high calories,
adequate protein, fat, plenty of fluids, minerals and
vitamins are to be given.
Care of the bladder -
The patient is encouraged to pass urine following delivery
as soon as convenient. If the patient
fails to pass urine, catheterisation should be done.
Catheterisation is also indicated in case of
incomplete emptying of bladder.
Care of the bawel -
The problem of constipation is much less because of early
ambulation and liberalisation of the
dietary intake. A diet containing sufficient roughage and
fluids is enough to move the bowel.
If necessary, mild laxative such as Igol (isopgol husk) two
teaspoons may be given at bed time.
Sleep -
The patient is in need of rest, both physical and mental. So
she should be protected against
worries and undue fatigue. Sleep is ensured providing
adequate physical and emotional support.
Care of the vulva and episiotomy -
Shortly after delivery, the vulva and buttocks are washed
with soapwater down over the anus
and a sterile pad is applied. The patient should look after
personal cleanliness of the vulval
region. The perineal wound should be dressed with spirit and
antiseptic power after each act
micturition and defaecation or at least twice a day.
Care of the breast -
The nipple should be washed with sterile water before each
feeding. It should be cleaned
and kept dry after the feeding is over. Nipple soreness is
avoided by frequent short feeding
rather than the prolonged feeding, keeping the nipple clean
and dry.
Maternal-infant bonding -
It starts from first few moments after birth. This is
manifested by fording, kissing, cuddling
and gazing at the infant. The baby should be kept in her bed
or in a cot besides her bed.
This is not only establishes the mother-child relationship
but the mother is conversant with
the art of baby care so that she can take full care of the
baby while at home.
Asepsis and antiseptic -
Asepsis must be maintained specially during the first week
of puerperium. Liberal use of
local antiseptics, aseptic measures during perineal wound
dressing, use of clean bed linen
and clothing are positive steps.
Immunization -
Administration of anti-D-gamma globulin to unimmunized
Rh-negative mother bearing
Rh-positive baby. The booster dose of tetanus toxoid should
be given at the time of
discharge, if it is not given during pregnancy.
118. Pregnancy and
congenital heart diseases.
Management of specific cardiac lesions in pregnancy
1. Atrial septal defect. This is
the most common heart lesion
complicating preg nancy.
- Hemodynamic effects. Most
patients are asymptomatic, and the
lesion is discovered by auscultation of a murmur. The
left-to-right
shunting will cause an increase in pulmonary blood flow, but
pulmonary hypertension is a relatively late finding.
- Effect of pregnancy. In
the absence of pulmonary hypertension,
this lesion is well tolerated in pregnancy and should not
threaten
maternal health. If the patient has experienced pulmonary
hypertension, then she is at risk for Eisenmenger's syndrome,
a
diagnosis that carries a maternal mortality of 50%.
Therefore, in
counseling the patient with an atrial septal defect (ASD),
it is critical
to evaluate her for pulmonary hypertension either by
noninvasive
testing such as an echocardiogram or by right-sided heart
catheterization.
2. Ventricular septal defect
- Hemodynamic effects. The
size of the defect determines the
degree of disability. A small lesion is hemodynamically
insignificant
and is well tolerated. With a large defect with significant
left-to-right
shunting, the initial abnormality would be left ventricular
hypertrophy
to compensate for the decrease in forward flow followed by
the
development of pulmonary hypertension caused by the
increased
pressures delivered to the pulmonary vasculature and,
finally,
biventricular hypertrophy with the right side pumping
against a
significant afterload.
- Effect of pregnancy. The
impact of pregnancy will depend on
the hemody namic abnormalities of the individual patient.
Without
pulmonary hyper tension, the decrease in systemic vascular
resistance caused by pregnancy may be beneficial, causing an increase in forward
flow. However, the in crease in blood volume may lead to congestive heart
failure in the patient with long-standing left ventricular hypertrophy. When
there is preexisting pulmonary hypertension, Eisenmenger's syndrome may
develop, a diagno sis that carries a risk of maternal morbidity of 50%. The
patient with an uncomplicated repaired ventricular septal defect (VSD) is not
at risk
during pregnancy.
- Management during pregnancy. In the absence of
pulmonary
hypertension, there are no special requirements during
pregnancy
except for bacterial endocarditis prophylaxis. With
pulmonary
hypertension, the management should be as for any patient at
risk for
Eisenmenger's syndrome.
3. Coarctation of the aorta is frequently associated with
other cardiovascular abnormalities such as bicuspid aortic
valve and
cerebral berry aneurysms.
- Hemodynamic effects. Systolic hypertension is
common
secondary to the obstruction to outflow, and there is
compensatory left
ventricular hyper trophy.
- Effect of pregnancy. With uncomplicated coarctation
of the aorta,
preg nancy is usually well tolerated. A minority of cases
will be
complicated by congestive heart failure secondary to
long-standing
left ventricular hypertrophy and dysfunction. There may be
an
increased risk for aortic dissection during pregnancy
secondary to the
effect of maternal hormones on the blood vessel wall
architecture.
- Fetal effects. Fetal growth may be affected by a
relative decrease in
blood flow to the maternal lower body, including the
placenta. The
risk for congen ital heart disease in offspring is
approximately 20%.
- Management during pregnancy. Hypertension should be
controlled to de crease the risk for aortic dissection and
rupture.
Bacterial endocarditis prophylaxis is recommended.
4. Tetralogy of Fallot describes
a syndrome of pulmonary
stenosis, right ventric ular hypertrophy, and large
ventricular septal
defect with an overriding aorta. This is the most common
cyanotic
lesion complicating pregnancy. The vast majority of pregnant
women
will have already had full or partial surgical repair,
because the
disease is otherwise usually fatal in childhood or early
adulthood.
- Hemodynamic effects. There is right outflow
obstruction with
resultant right-to-left shunting. This delivers blood with
decreased
oxygen satura tion to the systemic circulation. Right
ventricular
hypertrophy will develop as a result of the right outflow
obstruction.
- Effect of pregnancy. Pregnancy is poorly tolerated
in patients who
have not had surgical repair, because the decrease in
systemic vascular
resis tance will cause an increase in right-to-left shunting
with further
desatura- tion of blood flowing to the systemic circulation.
Patients
believed to have an especially poor prognosis in pregnancy
are those
with a baseline hematocrit greater than 60%, arterial oxygen
saturation
of less than 80%, a history of syncopal episodes, and
significant right
ventricular hypertension. Patients with a full surgical
repair before
pregnancy are not at increased risk during pregnancy.
Patients with
palliative (partial) repairs such as the Blalock- Taussig
(anastomosis
of the subclavian artery to the right pulmonary artery),
Potts
(anastomosis of the descending aorta to the left pulmonary
artery), or
Waterston-Cooley (anastomosis of the ascending aorta to the
right
pulmonary artery) may be minimally symptomatic before
pregnancy
and yet may be at risk for decompensation during pregnancy.
Therefore, it is important to evaluate functional status by
the
parameters just men tioned.
- Fetal effects. The fetus is at risk for growth
retardation as a result
of poor oxygen delivery. The risk for congenital heart
disease in
offspring is approximately 15 to 20%.
- Management during pregnancy. It is essential to
avoid
hypovolemia so as not to further obstruct outflow and
increase rightto-
left shunting and to supplement oxygen as necessary.
5. Aortic stenosis may be
due to congenital or acquired heart
disease. The most common congenital lesion is a bicuspid
valve, and
there frequently are other associated cardiovascular
anomalies. The
lesion may not be stenotic at birth but will become
progressively
stenotic with aging.
- Hemodynamic effects. When
the valve reaches a critical
narrowing in area of less than 1 cm2, there is obstruction
to left
ventricular outflow with development of left ventricular
hypertrophy.
This, in turn, may result in a fixed stroke volume and
ischemia as a
result of poor perfusion of the coronary blood vessels. With
additional
stress, the patient may decompen sate, with development of
left
ventricular failure.
- Effect of pregnancy. Pregnancy
is poorly tolerated in the patient
with critical aortic stenosis. The patient has a fixed
stroke volume and
is only able to increase cardiac output to meet the
increased demands
of pregnancy by increasing heart rate. An increase in heart
rate will
~ 166 ~
relatively decrease the diastolic portion of the cardiac
cycle, and this
will result in decreased time for coronary perfusion and
ventricular
filling. The risk of maternal mortality with critical aortic
stenosis is
approximately 15%; therefore, it is important to discuss and
offer
termi nation of pregnancy with severe degrees of disease.
- Fetal effects. There is
an increase in perinatal mortality primarily
related to maternal complications. The risk for congenital
heart
disease in off spring is approximately 20%.
- Management during pregnancy. In a patient with critical aortic
stenosis, strict limitation of activity is essential to
minimize the need
for cardiac output augmentation during pregnancy. It is very
important
to avoid situa tions that will further impede venous return
such as
hypovolemia. In addition, use of support hose may help to
maintain
venous return. Left- sided heart failure should be treated
as in the
nonpregnant patient. Bacte rial endocarditis prophylaxis is
recommended. In patients who have had a valve replaced, if
the valve
is mechanical, anticoagulation is required. Because warfarin
is
contraindicated during the first and third trimesters of
pregnancy, the
patient is normally maintained on therapeutic levels of
heparin for the
duration of pregnancy.
6. Pulmonary stenosis. Unlike
aortic stenosis, this lesion is not
usually progres sive.
- Hemodynamic effects. Mild
degrees of pulmonic stenosis usually
cause no symptoms or hemodynamic compromise. With severe
stenosis there will be obstruction to right ventricular
outflow, causing
the development of right ventricular hypertrophy.
- Effect of pregnancy. Most
patients with pulmonic stenosis tolerate
preg nancy well. However, the patient with severe stenosis
may be
unable to meet the demands for augmented cardiac output and
may not
tolerate the volume expansion of pregnancy, leading to congestive
heart failure.
- Fetal effects. Congenital
cardiac lesions are seen in approximately
20% of offspring.
- Management during pregnancy. Hypervolemia and hypovolemia
should be avoided. Right-sided heart failure should be
treated as in the
nonpregnant patient. If the patient fails medical therapy, a
surgical
valvotomy may be performed safely during pregnancy.
Bacterial
endocarditis prophylaxis is recommended.
119. Newborn care.
Postnatal care
CARE OF INFANT
Post delivery
1. Apgar score (see Neonatal resuscitation).
2. Keep baby warm and dry.
3. Encourage skin-to-skin contact.
4. Check birthweight, body temperature and head
circumference within 1 h.
5. Encourage initiation of breastfeeding within 1 h.
6. Offer IM vitamin K (orally if declined).
7. Ensure passing urine, and meconium passed within first 24
h.
Within 72 hours
Full neonatal examination (often completed within 48 hours):
1. Colour, breathing, behaviour, activity, posture, tone.
2. Head circumference. Check fontanelles, palate, nose,
ears, symmetry of head and facial
features, assess eyes (red reflex).
3. Limbs: proportions, symmetry, no. of digits, congenital
dislocation of the hip.
4. CVS: HR, murmurs, arrythmias.
5. Chest: auscultation, symmetry, signs of respiratory
distress, respiratory rate.
6. Abdomen: assess for organomegaly, check umbilical cord
insertion site.
7. Genitalia: exclude undescended testes in males.
8. Anus: exclude imperforate anus.
9. Spine: exclude spina bifida.
10. Note any birthmarks.
Assessment at 30 Seconds of Life. Apnea, gasping
respirations,
or heart rate < 100 bpm beyond 30 seconds after delivery
should prompt administration of positive-pressure
ventilation
with room air. Assisted ventilation rates of 30 to
60 breaths per minute are commonly employed, and the percent
of oxygen saturation is monitored by pulse oximetry. At
this point, supplemental oxygen can be given in graduated
increasing percentages to maintain oxygen saturation (Spo2)
values within a normal range (Vento, 2011). Adequate
ventilation
is indicated by improved heart rate.
After the first hour of life, newborns should receive eye
care, vitamin K, and recommended immunizations (birth dose of OPV and Hepatitis
B vaccine). They should be assessed for birth weight, gestational age,
congenital defects and signs of newborn illness. Special care should be
provided for sick newborns, those who are preterm and/or low birth weight, and
those who are exposed or infected by HIV or have congenital syphilis.
120. Perineal
dissection. Indications. Management after delivery.
121. Management of
breech delivery.
122. Retained parts of
placenta (causes, clinic, management).
123. Essential
hypertension and pregnancy.
Chronic hypertension (with or without renal
disease) existing prior to pregnancy can predispose to
the later development of superimposed pre-eclampsia.
Even in the absence of superimposed pre-eclampsia,
chronic hypertension is associated with increased
maternal and fetal morbidity and pregnancies
complicated by chronic hypertension should therefore
be regarded as high risk.
In mild cases (
150/100 mmHg) there is no
immediate indication to treat; however, the pregnancy
should be monitored carefully to detect rising blood
pressure or features of pre-eclampsia, as well as FGR
by serial ultrasound scans. Women who are receiving
antihypertensive medication before pregnancy are
often able to discontinue this for the fi rst part of
pregnancy because of the physiological fall in blood
pressure at this time. Some antihypertensives, such as
angiotensin-converting enzyme inhibitors, should be
discontinued because of the fetal risk.
If the blood pressure is consistently noted to
be
150/100 mmHg, antihypertensive medication
will need to be introduced or recommenced. This
is to reduce the risk of severe hypertension and the
attendant risks of intracerebral haemorrhage, although
treatment does not prevent placental abruption or
superimposed pre-eclampsia, nor infl uence perinatal
outcome. Preferred antihypertensive agents include
methyldopa (centrally acting agent) – which is
generally well tolerated, especially if women are
forewarned about the common transient side effects of
lethargy and postural hypotension – labetolol (alphaand
beta-blocker), and nifedipine (calcium-channel
blocker). The aim of antihypertensive medication is to
maintain the blood pressure below 160 mmHg systolic
and 100–110 mmHg diastolic.
The obstetric management of pre-existing
hypertension involves close monitoring for the
development of superimposed pre-eclampsia, which
may present with elevated blood pressure, new-onset
or worsening proteinuria, as well as the development
of FGR. Each case must be individually assessed,
but early delivery is rarely indicated unless preeclampsia
develops or the blood pressure is very
diffi cult to control. In general, it is reasonable to
await spontaneous labour or attempt vaginal delivery
by induction of labour (at around 38 weeks), ensuring
the maternal blood pressure is well controlled.
Post-natally, the maternal blood pressure will often
decrease, but careful observation is required in
the fi rst 48 hours because blood pressure tends to
increase again on the third or fourth postpartum
day. Breastfeeding is encouraged and although some
antihypertensive medication may enter the breast
milk, the standard antihypertensive medications are
not contraindicated in breastfeeding mothers.
124. Prenatal education
classes.
A series of classes, either online or in person,
provided for groups of pregnant women and
their partner or support person.
What types of prenatal education
are out there?
• Generic
• Specific to the stage
of pregnancy
• Refresher
• Programs for specific
groups
– Adolescents / Young
– Cultural groups
– Multiple pregnancy
– Diverse family structures
• Online
• Blended
• Drop-in
• One-to-one
• Other
– Transition to parenting
– Centering pregnancy
– Bereaved parents
Is prenatal education effective?
• Effectiveness in increasing the initiation of
breastfeeding.
• Effectiveness in improving specific health
behaviours such as hygiene practices to
reduce toxoplasmosis and follow-up sickle
cell screening.
• Research linking prenatal education to other
health behaviours has been inconclusive due
to selection bias or limited evidence.
What should be taught?
Organized in six main topics, with sub-topics:
– Prenatal health
– Physical and emotional preparation for childbirth
– Interventions to support, comfort and provide pain
management during labour and birth
– Physical, emotional and social needs of the new
family
– Breastfeeding
– The newborn
125. Maternity leave.
Maternity leave is a temporary period of absence from
employment granted to expectant or new mothers during the months immediately
before and after childbirth. These policies are generally aimed at supporting
the mother's full recovery from childbirth and facilitating a stronger
mother-child bond.
126. Course and
management of second stage of labor.
The second stage
This lasts from full dilatation of the cervix to delivery.
Descent, flexion and rotation are completed and followed
by extension as the head delivers.
The passive stage lasts from full dilatation until the head
reaches the pelvic floor and the woman experiences the
desire to push. Rotation and flexion are commonly
completed. This stage may last a few minutes, but can
be much longer.
The active stage is when the mother is pushing. The
pressure of the head on the pelvic floor produces an
irresistible desire to bear down, although epidural
analgesia
may prevent this. The woman gets in the most
comfortable position for her, but not supine, and pushes
with contractions. The fetus is delivered, on average,
after 40 minutes (nulliparous) or 20 minutes (multiparous).
This stage can be much quicker, but if it takes
>1 h spontaneous delivery becomes decreasingly likely.
Clinical Management of the Second Stage
As in the first stage, certain steps should be taken in the
clinical
management of the second stage of labor.
Maternal Position. With the exception of avoiding the supine
position, the mother may assume any comfortable position for
effective bearing down. If the birth is to occur in another
room,
primiparous patients should be moved at or before the
beginning of crowning. Multiparous patients should be brought to the delivery
room at the time of complete cervical dilation.
Bearing Down. With each contraction, the mother should be
encouraged to hold her breath and bear down with expulsive
efforts. This is particularly important for patients with regional anesthesia
because their reflex sensations may be impaired.
Fetal Monitoring. During the second stage, the fetal heart
rate
should be monitored continuously or evaluated every 5
minutes in
patients with obstetric risk factors. Fetal heart rate
decelerations (head compression or cord compression) with recovery following
the uterine contraction may normally occur during this stage.
Vaginal Examination. Progress should be recorded
approximately every 30 minutes during the second stage.
Particular attention should be paid to the descent and flexion of the
presenting part, the extent of internal rotation, and the development of
molding or caput.
127. Dropsy of
pregnancy. Clinic, diagnostic, management.
Dropsy pregnant (swelling pregnant) is one of the forms of
toxicity second half of pregnancy, characterized by swelling in the absence of
protein in the urine and normal blood pressure. Usually occurs after 28-30
weeks of pregnancy, often multiple. Edema is due to violation of water-salt
metabolism and blood circulation in the system of capillaries and
precapillaries in the result of changes in neuroendocrine regulation. In the
development of dropsy pregnant plays the role of a previous state of the body
(metabolism with a tendency to be overweight, liver disease and other).
Clinic
Clinical presentation and course. The disease develops more
often gradually. Before the appearance of visible swelling may occur the
so-called signal symptoms: excessive weight gain during the week (more 250-400
grams), symptoms of the "ring" (the ring on your finger becomes
logopedija), "tight shoes" (worn shoes becomes close). If timely
measures are not taken, visible swelling. There are 4 the prevalence of edema:
1) only swelling in the feet and lower legs; 2) swelling of the lower
extremities, the lower part of the abdomen and lumbar-sacral region (if the
woman is); 3) in addition to these, edema on the hands, pastos person; 4)
General swelling. The skin becomes glossy look, saving, however, normal color
(no pallor observed in renal edema, cyanosis, as edema associated with heart
disorder). Even when pronounced edema of cardiac activity, liver, kidneys is
not observed. There are no also accumulation of fluid in the cavity (pleural
and abdominal). In a period of growth swelling observed decrease of urine output
(negative diuresis), reducing its proportion. The General condition of the
pregnant remains satisfactory.
Forecast at a dropsy pregnant is usually favorable, timely
treatment gives good results, pregnancy born at the end and ends with
childbirth. After delivery dropsy pregnant disappears. In rare cases, if the
prevention and treatment has not been organised properly, the disease can move
rapidly in nephropathy pregnant and even eclampsia.
Management and Prevent
When the signal symptoms dropsy pregnant midwife should
refer the pregnant woman to the district hospital for consultation. At
diagnosis dropsy pregnant during this period it is recommended diet with
restriction of salt (not more than 2 g / day), reduce the amount of fluid (not
more than 800 - 1000 ml per day). A careful monitoring (every week, sometimes
more often!) weight, blood pressure, repeated analyses of urine. Action taken
in time can prevent development of toxemia.
With the development of explicit edema treatment should be
carried out in the hospital. Only in exceptional cases, if there is confidence
that the patient will perform all assignments and homes can provide good care,
treatment can be performed at home under surveillance antenatal clinic.
Prescribe diuretics - gipotiazid (25 mg 2 times in the morning at four-hour
intervals) 3 consecutive days, for the night sedatives: motherwort tincture at
the rate of 10 g to 200 g of water on 1 table, HP, papaverine 0.02 g, better in
candles (for 1 suppository 2 times a day). The woman should stay in bed (better
defined the diuretic effect). Once a week, spend a day of fasting (fluid
restriction to 800 ml and salt to 2 g per day) and after poluraspredelenia
(without the first liquid dish). Prescribe vitamins (vitamin C, vitamins of
group b). If within 3-4 days of improvement does not occur, then treated as if
nephropathy pregnant.
Prevention. Careful monitoring and active patronage of
pregnant women, monitor their observance of hygiene, especially diet. Cm. also
Pregnancy.
Diagnosis and Tests
To check for edema that
is not obvious, you can gently press your thumb over the foot, ankle or leg
with slow, steady pressure. If edema is present, an indentation will show
on the skin. A professional evaluation to determine the cause of leg
swelling is needed. If both legs are swollen, your doctor will inquire
about other symptoms and perform a physical examination. A urine test
will show whether you are losing protein from the kidneys.
Blood tests, a chest X-ray and an electrocardiogram (ECG)
may be ordered.
128. Aseptic and
antiseptic in obstetrical practice.
Proper client preparation with antiseptics before an
obstetric procedure, such as a cesarean section or uterine evacuation, involves
applying
an antiseptic solution to the client’s skin, vagina, cervix,
or perineum
to destroy or prevent the growth of microorganisms. Most
surgical-site infections result from contamination during
surgery—
not, as many people believe, because clients do not keep the
wound
clean after surgery. Frequently, infections are caused by
bacteria
from the client’s skin or tissues. Therefore, proper client
prep before a procedure is critical.
Common antiseptics used for client prep:
• An iodophor (e.g., Betadine)
• 4% chlorhexidine gluconate (e.g., Hibiclens)
• Chlorhexidine gluconate with cetrimide (e.g., Savlon)
• 1–3% iodine, followed by 60–90% alcohol (ethyl or
isopropyl). Note:
Alcohol and alcohol-based antiseptics should be used on the
skin
only; do not use them to prepare the vagina, cervix, or
perineum.
Provide the following immunization to any woman who
had an unhygienic delivery or an unsafe termination of
pregnancy:
• If she has already been immunized, give a booster
injection of tetanus
toxoid 0.5 mL IM.
• If she has not been immunized, give antitetanus serum
1,500 units IM
immediately, and tell her to return in four weeks for a
booster injection
of tetanus toxoid 0.5 mL IM.
When the use of prophylactic antibiotics is indicated for
emergency obstetric care:
• Use broad-spectrum antibiotics (e.g., ampicillin,
cephalosporins, or
combination antibiotics) that are effective against the
microorganisms
most likely to cause infections.
• Whenever possible, give prophylactic antibiotics before or
during the
procedure, not afterward. (During cesarean sections,
administer broadspectrum
antibiotics immediately after clamping the cord.)
• Give only a single dose of antibiotics, unless surgery is
prolonged (more
than 6 hours) or blood loss is excessive (more than 1.5 L).
Aseptic Technique
“Aseptic technique” refers to the practices performed just
before or
during a clinical or surgical procedure to reduce the
client’s risk of
infection by reducing the likelihood that microorganisms
will enter
areas of the body where they can cause infection.
Aseptic technique includes:
• Surgical scrub
• Using physical barriers (such as gloves and other surgical
attire,
surgical drapes, and pads)
• Client prep
• Establishing and maintaining a sterile field
• Good surgical technique
• Appropriate use of prophylactic antibiotics
• Creating a safer surgical/procedure area
129. Management of acute
anemia in obstetrical practice.
130. Mechanism of
delivery on flat pelvis.
Check question 114
131. Methods of
estimating fetal weight before labor.
Tactile assessment
a) Clinical Methods: In
clinical methods tactile assessment
of foetal size, clinical
risk factor estimation, Maternal
self estimated foetal
weight and Prediction equations of
birth weight are included.
b) Imaging Methods: This
includes ultrasonography and
magnetic resonance imaging.
Some investigators
consider sonographic estimates
to be superior to clinical
estimates others confer
similar level of accuracy.
Several studies indicate
that physician conducted
physical examination of
pregnant women and estimated
foetal weight are superior
to ultrasonic foetal
measurement (3-7,12-46).Williams
textbook concludes
that estimation of foetal
weight from ultrasonic
measurements is not proven
to be reliable(47).It even
carries a risk of
sonologically induced chromosomal
anomalies.
Dare et al used this technique by multiplying the abdominal
girth (cm) with symphysiofundal height (cm) and calculated
the
estimated foetal weight in grams (21).However, this is less
accurate for obese than non obese and carries a significant
intra
observer variation. The inherent growth potential of the
baby and
nutritional status of the mother are concurrently measured.
The
resultant estimate is closest to the actual birth weight as
pointed
by several prospective studies (1, 26, 27, 29, 34).
Clinical risk Factor
This method involves quantitative assessment of clinical
risk
factors and has been shown to be valuable in predicting
foetal
weight. In case of foetal macrosomia ,the presence of risk
factors
,such as maternal diabetes mellitus ,prolonged pregnancy,
obesity
,pregnancy weight gain of >20 kg, maternal age >35
years,
maternal height> 5ft 3 inches, multiparity, male foetal
sex and
white race should be added. In low estimated birth weight
socioeconomic status, constitutionally small mother, poor
maternal weight gain, foetal infections, congenital
malformations, chromosomal abnormality, teratogenic
exposure,
maternal anaemia, Anti phospholipid Antibody syndrome and
other medical disorders complicating pregnancy should be
mentioned.
Maternal Self
estimation
In literate society maternal self estimation of foetal birth
weight in multiparous women show comparable accuracy to
clinical palpation in some studies for predicting abnormally
large
foetus (24,29).
Birth weight Prediction
equations
Various calculations and
formulae based on measuring
uterine fundal height above
symphysis pubis have been
developed. Ojwang et al
used the product of symphysiofundal
height and abdominal girth
measurement at various levels in
centimetres above symphysis
pubis in obtaining a fairly
acceptable predictive value
but with considerable variation from
the mean(20).Dare et al
simplified and used the product of
symphysiofundal height(Mc
Donald’s measurement) and
abdominal girth at the
level of umbilicus measured in centimetres
and result expressed in
grams to estimate foetal weight in uteru at
term ,and the estimation
correlated well with birth weight (21).
Johnson’s formula for
estimation of foetal weight in vertex
presentation is as follows
Foetal weight (grams) = (Mc Donald’s
measurement of
symphysiofundal height in
cm –X) x 155 where X = 13, when
presenting part was not
engaged, X = 12 when presenting part is
at 0 station and X = 11
when presenting part was at +1 station. If
a patient weighs more than
91 kg, 1cm is subtracted from the
fundal height.
Dawn’s formula states that weight (grams) =
longitudinal
diameter of the uterus x
transverse diameter of the uterus x
1.44/2 .Measurements are
made with pelvimeter. Double
abdominal wall thickness
was also measured pelvimeter. If
Double abdominal wall
thickness was more than 3 cm, the excess
was deducted from the
longitudinal diameter
obstetric
ultrasonography
MRI
132. Blood transfusion
in obstetric practice.
133. Ruptures of cervix (causes, diagnostic, management).
134. Medical
indications for artificial abortion.
A The continuance of the pregnancy would involve risk to
the life of the pregnant woman greater than if the pregnancy
were terminated
B The termination is necessary to prevent grave permanent
injury to the physical or mental health of the pregnant
woman
C The pregnancy has not exceeded its 24th week and that
the continuance of the pregnancy would involve risk,
greater than if the pregnancy were terminated, of injury to
the physical or mental health of the pregnant woman
D The pregnancy has not exceeded its 24th week and that
the continuance of the pregnancy would involve risk,
greater than if the pregnancy were terminated, of injury to
the physical or mental health of any existing child(ren) of
the family of the pregnant woman
E There is a substantial risk that if the child were born it
would suffer from such physical or mental abnormalities
as to be seriously handicapped
Maternal
Fetal
135. Cephalohematoma.
136. Craniotomy.
Indications. Conditions. Technique.
Definition
It is an operation to make a perforation on the fetal head ,
to evacuate thecontent followed by extraction of the fetus.
Indications
* Cephalic
presentation producing obstructed labour with dead fetus
*
Hydrocephalus even in a living
fetus – this is applicable for bith forecoming and aftercoming head .
* Interlocking
head of twins
Condition to be full filled
® The cervix must
be fully dilated
® Baby must be dead
Contraindication
® The operation
should not be done when the pelvis is severly contracted. So as to shortened
the true conjugate to less than 7.5cm (3``). In such condition the baby cannot
be delivered as the bimastoid diameter 7.5cm which cannot be compressed.
® Rupture of the
uterus were laprotomy is essential
Procedure
Step1
Two fingers are introduced in to the vagina and the finger
tips are to be planned on proposed site of perforation. However when the suture
line cannot be defined because of big caput , the perforation should be done
through the dependent part.
Site of preparation
Vertex:- on the parietal bone either side of the sagittal
sutures is avoided to prevent collapse of the bone thereby preventing escape of
the brain matter
Face :- through the orbit or hard plate
Brow :- through the
frontal bone
Step 2
The Oldham’s perforator , with the blades closed , is
introduced under the palmaraspect of the fingers protecting the anterior
vaginal wall and the adjacent bladder until the tip reaches the proposed site
of perforation.
Step 3 :- By rotating movements the skull is perforated .
during this step, an assistant is asked to steady the head per abdomen in a
manner of first pelvic grip. After the skull is perforated , the instrument is
thrust up to the shoulder and the handles approximated ,so as to allow
separation of the sharp blades for about 2.5 cm .
The blades
are again apposed by separating the handles. The instruments is brought out
keeping the tip of the blades still inside the cranium the instruments is
rotated at right angle and then again threst inup to the shoulders. The handles
are once more to be compressed of as to separate the blades for about 2.5cm.
The perforator area now looks like a cross. The instruments with the blades
closed is then thrust in beyond the guard to churn the brain matter. The
instruments with the blades closed , is brought out under the guidance of the
two fingers still placed inside the vagina.
Alternative to Oldham’s perforator , similar procedure could
be performed using a sharp pointed Mayo’s scissors.
Step 4 :- With the fingers brain matter is evaluated. The
idea is to make the skull collapsed as much as possible.
Step 5 :-when the skull is found sufficiently compressed,
the extraction of the fetus is achieved either by using cranioclast or by two
Gaint Vulsella are used to hold the incised skull and scalp margins.
Step 6 :- the traction is now excreted in the same direction
is like that mentioned in forceps operations.
Step 7 :- after the delivery of the placenta, the
uterovaginal canal must be explored as a routine for evidence of rupture uterus
or any tear.
Inj. Methergin 0.2mg is to be given intravenously with the
delivery of the anterior shoulder. The rest of the delivery is completed as in
normal delivery.
Forceps Vs craniotomy in a dead fetus
If the delivery of the uncompressed head can be accomplished
with out much force with consequent injuries to the mother, forceps delivery is
preferred. But if it is found difficult and damaging to the mother, craniotomy
is safer.
137. Signs of preterm
infant.
138. Structure of
maternity hospital.
Minsk health care institution “City
clinical maternity hospital №2» provides highly qualified obstetric-gynecologic
help for women and newborns of Minsk. Our maternity hospital is a perinatal
center of the third level, a premature delivery management center.
The institution has the following
structure:
·
an
obstetric-gynecologic in-patient department where both term and premature
deliveries take place, newborns are treated and cared for, and gynecologic
surgeries are performed, including laparoscopic surgeries. About 6 000
deliveries take place in the maternity hospital during one year, and 125
thousands children were born at the maternity hospital over more than 30 years;
·
an interdistrict
center of perinatal ultrasound diagnostics where screening ultrasound examinations
during the pregnancy are performed. More than 25000 pregnant women are examined
in the center during one year. About 9000 gynecologic examinations, up to 4000
thyroid gland, mammary glands, abdominal cavity organs, man’s urogenital system
examinations are performed in the ultrasound diagnostics department of the
maternity hospital. The examinations are performed with the help of the
advanced equipment by qualified specialists;
·
a family planning
city center where infertile married couples, women with recurrent pregnancy
loss undergo therapy, and а preparation for the delivery with the partner is
performed. More than 1000 couples have become happy parents over the
years of center existence.Since 2004 a full course of infertility
treatment has been held, including additional reproductive techniques (IVF and
others). About 400 couples are trained at partner delivery courses.
139. Neonatal injuries
associated with labor.
Common Birth-Related
Traumas
Generally, the most common
neonatal injuries affect a baby’s head, neck, and shoulders, although they can
cause damage to any other part of the body. These areas of the body are more
likely to be injured because most babies are born in a head-first position.
According to the Packard Children’s Hospital, the most common traumatic
injuries include:
Caput Succedaneum
Caput succedaneum is a
condition marked by scalp swelling, typically during or shortly after birth. It
is usually caused by pressure from the mother’s uterus or vaginal wall during
delivery. Bruising of the scalp is more likely to happen during a long and
difficult labor, especially in situations when the amniotic sac has broken and
the baby’s head is unprotected while passing through the birth canal.
Caput succedaneum can also
be caused by the use of vacuum extraction devices during a protracted delivery.
Cephalohematoma
Cephalohematoma is an
accumulation of blood below the baby’s periosteum, the protective membrane that
covers an infant’s skull. Cephalohematoma shows up as lumps on a baby’s head,
usually several hours after delivery. The lumps feel soft and may grow larger
during the baby’s first hours postpartum.
Most cephalohematomas do
not require medical attention and disappear within a few weeks or months as the
body reabsorbs the blood. However, some cephalohematomas may cause jaundice if
they are too large and too many red blood cells break down.
Bruising and Broken Bones
Bruising may occur on a
baby’s face, head, and/or other body parts due to the physical stresses of the
passage through the birth canal or contact with bones and tissue in the
mother’s pelvis. The use of forceps during delivery may also leave forceps
marks on a newborn’s head or face, especially when doctors use too much force.
In addition, vacuum extraction may cause lacerations or bruising on a baby’s
scalp.
Similar to bruising, broken
bones can occur with improper use of birth-assisting tools or when an infant is
tugged too forcefully. In extremely rare instances, a physician or someone on
the medical staff may drop a newborn.
Subconjunctival Hemorrhage
Subconjunctival hemorrhage
is bleeding that occurs when small blood vessels in the baby’s eyes break. It
may be present in one or both of the infant’s eyes and appears as a bright red
band surrounding the iris. Subconjunctival hemorrhages do not cause permanent
damage to the eyes. The red area vanishes within a matter of days as the body
reabsorbs the blood.
Bell’s Palsy
Bells’ palsy occurs when a
baby’s facial nerve is damaged during labor or birth. In most cases, nerve
damage is caused by pressure on the infant’s face during the passage through
the birth canal.However, facial paralysis can be also caused by doctors that
use forceps during delivery.
Nerve damage is most
noticeable when babies cry. The facial muscles on the side where the nerve was
injured can’t move, and the eye on that side remains open.
Bell’s palsy eventually
improves without treatment if the nerve is only bruised. If the baby’s facial
nerve is torn, surgery may be needed to restore muscular function on the
affected area.
Brachial Plexus Injury
A brachial plexus injury is
the result of an injury to a baby’s brachial plexus. This is a network of
nerves that connects the spinal cord to the baby’s arms and hands. Brachial
palsy is a common occurrence in difficult births, especially if a baby’s
shoulder gets stuck in the birth canal and a doctor tugs hard on one arm to
help extract the newborn.
The most common sign of
brachial palsy is when a baby can’t flex or rotate the affected arm. The
severity of the injury depends on how badly damaged the nerves are. If the
nerves are only bruised or stretched, the injury heals over a period of weeks
or months and arm movement is restored with the aid of physical therapy.
More serious injuries, in
which the nerves are torn, often result in permanent nerve damage.
Oxygen Deprivation
Oxygen deprivation, or
anoxia, before or during birth can cause serious health problems to a newborn.
This type of birth of trauma can occur if the placenta separates prematurely or
if the umbilical cord becomes entangled around the baby’s neck and reduces
oxygen flow to the brain.
Inadequate oxygen supply
often causes damage to a baby’s cerebellum, the part of the brain that controls
the body motor functions. This results in the onset of cerebral palsy (CP), a
group of neuromuscular disabilities that affect a child’s ability to control
movement, posture, and muscle tone.
Oxygen deprivation can also
occur if a baby doesn’t start breathing independently after birth. Delays in
breathing that last for 3 minutes or more are a high risk factor of serious
brain damage. This category of birth injury destroys brain cells within a
matter of minutes and causes seizures, coma, and, if a baby is not placed in
life support in time, death.
Oxygen deprivation causes
permanent disabilities like cerebral palsy, and is also a major cause of
hearing impairment, partial or total blindness, learning disabilities, and
other complications.
Hypoxia is a slightly less
severe form of oxygen-related birth trauma. Unlike anoxia, which is used to
describe total oxygen deprivation, hypoxia refers to low levels of oxygen in a
baby’s circulatory system.
Fractures
Fractures are the most
common injuries associated with birth trauma. Fractures generally affect a
baby’s clavicle (collarbone) and are frequently caused by shoulder dystocia or
during breech deliveries. This type of injury prevents a baby from moving the
arm on the affected side. If the infant feels pain as a result of the fracture,
a splint or soft bandage is needed to prevent jostling of the arm until the
injury heals.
Most birth traumas are
conditions that usually heal on their own without any medical treatment. Babies
often recover with few or no complications, although individual outcomes depend
on a wide range of factors, such as the severity and cause of the injuries.
In many instances, birth
trauma can be avoided if doctors recognize and foresee medical risk factors.
Proactive measures, such as monitoring the mother’s health or using ultrasound
images to check the fetus’ position in the weeks and days before labor, often
prevent help birth trauma and injuries.
140. Amniotic fluid
embolism.
141. Placenta accreta
and placenta percreta.
The placenta normally
attaches to the uterine wall, however there is a condition that occurs where
the placenta attaches itself too deeply into the wall of the uterus.
This condition is known as
placenta accreta, placenta increta, or placenta percreta depending on the
severity and deepness of the placenta attachment. Approximately 1 in 2,500
pregnancies experience placenta accreta, increta or percreta.
What is the difference between accreta, increta or
percreta?
The difference between
placenta accreta, increta or percreta is determined by the severity of the
attachment of the placenta to the uterine wall.
Placenta Accreta occurs
when the placenta attaches too deep in the uterine wall but it does not
penetrate the uterine muscle. Placenta accreta is the most common accounting
for approximately 75% of all cases. Placenta Increta occurs when the placenta
attaches even deeper into the uterine wall and does penetrate into the uterine
muscle. Placenta increta accounts for approximately 15% of all cases. Placenta
Percreta occurs when the placenta penetrates through the entire uterine wall
and attaches to another organ such as the bladder. Placenta percreta is the
least common of the three conditions accounting for approximately 5% of all
cases.
What causes placenta accreta?
The specific cause of placenta accreta is unknown, but it can be related
to placenta previa and
previous cesarean
deliveries. Placenta accreta is present in 5% to 10% of women with
placenta previa.
A cesarean delivery increases the possibility of a future placenta
accreta, and the more cesareans, the greater the increase. Multiple cesareans
were present in over 60% of placenta accreta cases.
What are the risks of placenta
accreta to the baby?
Premature delivery and subsequent complications are the primary concerns
for the baby. Bleeding during the third
trimester may be a warning sign that placenta accreta exists,
and when placenta accreta occurs it commonly results in a premature delivery.
Your healthcare provider will examine your condition and use medication, bed rest and
whatever else necessary to help continue the pregnancy towards full term.
What are the risks of placenta
accreta to the mother?
The placenta usually has difficulty separating from the uterine wall. The
primary concern for the mother is hemorrhaging during manual attempts to detach
the placenta. Severe hemorrhaging can be life threatening.
Other concerns involve damage to the uterus or other organs (percreta)
during removal of the placenta. Hysterectomy is a common therapeutic
intervention, but the results involve the loss of the uterus and the ability to
conceive.
What is the treatment for placenta
accreta?
There is nothing a woman can do to prevent placenta accreta, and there is
little that can be done for treatment once placenta accreta has been diagnosed.
If you have been diagnosed with placenta accreta your healthcare provider will
monitor your pregnancy with the intent of scheduling a delivery and using a
surgery that may spare the uterus.
It is particularly important to discuss this surgery with your doctor if
you desire to have additional children.
Unfortunately, placenta accreta may be severe enough that a hysterectomy
may be needed. Again, it is important to discuss surgical options with your
healthcare provider.
142. Congenital
anomalies.
Definition
Congenital
anomalies are also known as birth defects, congenital disorders or congenital
malformations. Congenital anomalies can be defined as structural or functional
anomalies (e.g. metabolic disorders) that occur during intrauterine life and
can be identified prenatally, at birth or later in life.
Causes
and risk factors
Although
approximately 50% of all congenital anomalies cannot be linked to a specific
cause, there are some known causes or risk factors.
Socioeconomic
and demographic factors
Although
low income may be an indirect determinant, congenital anomalies are more
frequent among resource-constrained families and countries. It is estimated
that about 94% of severe congenital anomalies occur in low- and middle-income
countries, where women often lack access to sufficient, nutritious food and may
have increased exposure to agents or factors such as infection and alcohol that
induce or increase the incidence of abnormal prenatal development. Further,
advanced maternal age increases the risk of chromosomal abnormalities,
including Down syndrome, while young maternal age increases the risk of some
congenital anomalies.
Genetic
factors
Consanguinity
(when parents are related by blood) increases the prevalence of rare genetic
congenital anomalies and nearly doubles the risk for neonatal and childhood
death, intellectual disability and other anomalies in first-cousin unions. Some
ethnic communities (e.g. Ashkenazi Jews or Finns) have a comparatively high
prevalence of rare genetic mutations, leading to a higher risk of congenital
anomalies.
Infections
Maternal
infections such as syphilis and rubella are a significant cause of congenital
anomalies in low- and middle-income countries.
Maternal
nutritional status
Iodine
deficiency, folate insufficiency, obesity and diabetes mellitus are linked to
some congenital anomalies. For example, folate insufficiency increases the risk
of having a baby with a neural tube defect. Also, excessive vitamin A intake
may affect the normal development of an embryo or fetus.
Environmental
factors
Maternal
exposure to certain pesticides and other chemicals, as well as certain
medications, alcohol, tobacco, psychoactive drugs and radiation during
pregnancy, may increase the risk of having a fetus or neonate affected by
congenital anomalies. Working or living near, or in, waste sites, smelters or
mines may also be a risk factor, especially if the mother is exposed to other
environmental risk factors or nutritional deficiencies.
Prevention
Preventive
public health measures delivered through health services decrease the frequency
of certain congenital anomalies. Primary prevention of congenital anomalies
includes:
·
improving the diet of women
throughout their reproductive years, ensuring an adequate dietary intake of
vitamins and minerals, and particularly folic acid, through daily oral
supplements or fortification of staple foods such as wheat or maize flours;
·
ensuring mothers abstain from, or
restrict, their intake of harmful substances, particularly alcohol;
·
controlling preconceptional and
gestational diabetes, through counselling, weight management, diet and
administration of insulin when needed;
·
avoiding environmental exposure
to hazardous substances (e.g. heavy metals, pesticides) during pregnancy;
·
ensuring that any exposure of
pregnant women to medications or medical radiation (e.g. imaging rays) is
justified, based on careful health risk–benefit analysis;
·
improving vaccination coverage,
especially against the rubella virus, for children and women. Rubella can be
prevented through childhood vaccination. The rubella vaccine can also be given
at least 1 month prior to pregnancy to women who have not been vaccinated and
do not have a history of rubella in childhood;
·
increasing and strengthening
education of health staff and others involved in promoting prevention of
congenital anomalies.
Detection
Health
care before (preconception) and around the time of conception (peri-conception)
includes basic reproductive health practices, as well as medical genetic
screening and counselling. Screening can be conducted during the 3 periods
listed next.
·
Preconception screening can be
useful to identify people at risk for specific disorders or at risk for passing
a disorder onto their children. Screening includes obtaining family histories
and carrier screening, and is particularly valuable in countries where
consanguineous marriage is common.
·
Peri-conception screening:
maternal characteristics may increase risk, and screening results should be
used to offer appropriate care, according to risk. This may include screening
for young or advanced maternal age, as well as screening for use of alcohol,
tobacco or other psychoactive drugs. Ultrasound can be used to screen for Down
syndrome during the first trimester, and for severe fetal anomalies during the
second trimester. Additional tests, and amniocentesis may help in the detection
of neural tube defects and chromosomal abnormalities during the first and
second trimesters.
·
Neonatal screening includes
clinical examination and screening for disorders of the blood, metabolism and
hormone production. Screening for deafness and heart defects, as well as early
detection of congenital anomalies, can facilitate life-saving treatments and
prevent progression towards some physical, intellectual, visual or auditory
disabilities. In some countries, babies are routinely screened for
abnormalities of the thyroid or adrenal glands before discharge from the
maternity unit.
Treatment
and care
Many
structural congenital anomalies can be corrected with paediatric surgery and
early treatment can be administered to children with functional problems such
as thalassaemia (inherited recessive blood disorders), sickle cell disorders
and congenital hypothyroidism (reduced function of the thyroid)
Note: For specific anomalies
http://www.slideshare.net/doc1811/congenital-malformations
143. Nausea and
vomiting at pregnancy. Clinic, diagnostic, management.
144. Threatened and
imminent abortion.
145. Diagnostic of
anterpartum asphyxia.
146. Premature rupture
of membranes. Causes. Complications. Prevention.
Prelabour rupture of membranes (PROM) at term
DEFINITION Spontaneous rupture of membranes prior to onset
of labour at term.
AETIOLOGY Natural physiological mechanisms including Braxton
Hicks contractions and
cervical ripening lead to weakening of the membranes.
ASSOCIATIONS/RISK FACTORS None known.
EPIDEMIOLOGY Affects 8% of pregnant women.
HISTORY Sudden gush of fluid loss PV, followed by constant
trickle.
EXAMINATION
General: Assess for signs of infection (fever, tachycardia).
Vaginal: Avoid if possible (" risk infection).
Speculum: (If history uncertain) confirm pooling of liquor
in vagina, note liquor colour.
PATHOLOGY/PATHOGENESIS NA.
INVESTIGATIONS
Microbiology: Consider HVS/LVS.
MANAGEMENT
Clear liquor and no known GBS
Expectant management for 24 hours (majority ofwomenwill
labour). Offer augmentation of
labour after 24 hours (may opt for expectant management for
up to 72 hours with 4-hourly
temperature and 24-hourly fetal monitoring). Augment labour
with prostaglandin or
oxytocin infusion. Antibiotic cover (benzylpenicillin or
clindamycin if penicillin allergic)
dependent on unit protocol.
Meconium or known GBS or pyrexia
Augment labour immediately (antibiotics if known
GBS/pyrexia).
Postnatal
Neonatal observation required for at least 12 hours.
COMPLICATIONS Increased risk of ascending infection.
PROGNOSIS Sixty percent of women will labour over the next
24 hours.
147. Threaten rupture
of uterus.
Threatening diagnosis of uterine rupture particularly difficult
in cases where signs of overdistension of the uterus are mild or absent. In
such cases it is necessary to be guided symptom of "filling of the
bladder," or the appearance of protrusions above the vagina (a consequence
of tissue edema in the bladder), unexpectedly coming asphyxia, the onset of
involuntary bearing-down activity with high standing head, etc. However, these
symptoms are often absent or are expressed extremely weak.
The appearance of pregnant women who have had previous
cesarean section before the onset of labor (or during birth) bleeding from the
uterus, sometimes accompanied by pain in the abdomen or in the uterus (in the
old scar), should be seen as signals of a possible spread of incipient uterine
scar tissue.
In the event of rupture of the uterine scar are not only the
morphological change of the tissues, but the germination of the elements of
trophoblast in the uterine wall at the former site of the incision.
Behavior of mothers with a typically flowing threatening
uterine rupture is very characteristic: it is torn in the bed all the time
moaning, begging for help, complaining about "bursting" pain in the
abdomen, which is observed both outside and during the fight. Face red,
frightened. Tongue and lips dry. Pulse and respiration are increasing
dramatically. The body temperature usually rises, due to excessive muscular
work of the uterus.
In some cases, a woman in labor at times even lose
consciousness. The pressure of the presenting part on the bladder causing more
frequent urination. The woman in this state, reflexively straining, which, of
course, only accelerates the onset of uterine rupture.
In threatening uterine rupture should immediately stop
further overstretching of the uterus, which is partly achieved by moving the
pregnant woman to the position on the side opposite to the existing position of
the fetus. Assigned injection pantopon, magnesium sulphate and deep inhalation
anesthesia in order to complete shutdown of labor for the time necessary to
prepare for the operation.
Method of delivery should be carefully and gentle.
Significant role in the choice of a treatment is the situation and the
conditions in which the doctor works. Those surgical activities that can be
easily applied in clinics and hospitals (cervical or corporeal cesarean
section) are not always available in a GP practice. Where there are no
conditions for the production of C-section, the doctor is forced to resort to
the old methods of vaginal delivery — to the punch head with kranioklaziey
(when presenting the head) or decapitation, exenteration of the chest,
eventeratsii (with transverse or oblique position of the fetus). Said
operations should be carried out with great care, without much physical effort.
We must always remember that every action may cause grave gap cancer or help to
increase the size of an existing injury.
The method of choice for threatening uterine rupture is in
uninfected cases and with a live fetus cesarean section and only in exceptional
cases (if you can not make a C-section or stillbirth) — plodorazrushayuschie
operation.
With cephalic presentation (occipital, frontal, facial or
perednegolovnoe) perforation head dvuzubtsami captured by the skin, must be
carried out under the control of the mirrors, with subsequent destruction of
the brain with a spoon and scooping it out (washout).
When mobile (pressed) to fix her head at the entrance to the
pelvis through the abdominal wall should be very careful to avoid further
injury.
Must be very careful and overlay diaclast. Extraction of the
fetus should be slow and smooth, without the use of excessive force and carried
out in accordance with Biomechanism birth. During the traction necessary to
monitor the throat, while the edges as needed seasoned finger.
With the wide shoulder girdle is kleydotomiya, and sometimes
— and exenteration of the chest.
At birth in breech made subsequent perforation head.
In threatening uterine rupture in the event of running
transverse position fetal cephalic strictly prohibited even with a live fetus
and under deep anesthesia. Turn under such conditions usually lead to uterine
rupture and new complications.
In threatening uterine rupture, we absolutely are against
transporting women from one hospital to another, and we believe it necessary to
carefully rodorazreshayuschih her in this situation, at least with
plodorazrushayuschih operations. In the absence of conditions for delivery
on-site transportation is admissible as an exception mothers after shutdown of
labor (the injection of morphine, deep anesthesia, etc.) must accompany the
woman in childbirth doctor or midwife.
148. Physical
examinations of newborn in the delivery room.
Initial post-delivery examination
A brief screening examination should be conducted checking
the face, eyes, mouth, chest, abdomen, spine and limbs to exclude major
abnormalities. A strong cry and a widespread pink blush over the face and body
are good signs that all is well. Some children may be born with indiscriminate
genitalia. In such cases it is important not to guess at the likely gender of
the child, but advise that it is uncertain and that further tests will be
needed. If you have sufficient clinical experience, an orogastric tube should
be passed when the neonate's mother has suffered polyhydramnios. This excludes
oesophageal atresia.
The APGAR score
The Apgar
score gives a reproducible,
quantitative, semi-objective assessment of neonatal condition that is useful
for assessing a baby's progress or deterioration immediately after delivery.[2] It is important to document it
for medicolegal reasons. It is most useful following complicated births or
where there are unanticipated problems with the baby after delivery. It should
be checked at delivery and two and five minutes subsequently; these results
should be documented in the partogram, maternal and neonatal notes.
The Apgar Scoring System
|
|||
Assigned score
|
0
|
1
|
2
|
Colour
of baby
|
Blue,
pale
|
Body
pink, extremities blue
|
Completely
pink
|
Respiratory
effort
|
Absent
|
Weak
cry, hypoventilation
|
Good,
strong cry and adequate breaths
|
Muscle
tone
|
Limp
|
Some
flexion of extremities
|
Active
motion with extremities well flexed
|
Reflex
irritability (response to plantar stimulation)
|
No
response
|
Grimace
|
Cry
|
Heart
rate
|
Absent
|
Slow
(<100 bpm)
|
Fast
(>100 bpm)
|
There is an excess of mortality and an increased risk of
severe neurological morbidity in infants with total Apgar score <7 at five
minutes.[3]
Routine neonatal examination
Known colloquially as 'the baby check', this examination
should be carried out by a member of the paediatric team where the baby is
still in hospital, or by the GP and primary care team following home births.
The National Institute for Health and Care Excellence (NICE)[2] recommends that the aims of the
examination should be fully explained to the parent(s) before it is conducted.
The findings should be shared with the parent(s) and recorded in the postnatal
care plan and the personal child health record. NICE advises that the
examination should be carried out within 72 hours of birth and incorporate:
·
A review of parental concerns and the
baby's medical history
·
Family, maternal, antenatal and
perinatal history
·
Fetal, neonatal and infant history,
including any previously plotted birth weight and head circumference
·
Whether the baby has passed meconium
and urine (and urinary stream in a boy)
·
Other screening tests as recommended
by the UK National Screening Committee should also be carried out or arranged
at this time[1]
The examination is best conducted in
a well-lit, warm, private room with the mother in attendance and able to see
and help with what is being done.
Suggested schema for screening
neonatal examination
First
wash your hands thoroughly to reduce the risk of cross-infection. Then:
Listen and observe
·
Assess overall
appearance. Note general tone, sleepiness and
rousability. Observe general condition, proportions and maturity.
·
Look carefully for evidence of jaundice (preferably in
bright, natural light). Note whether there are any birthmarks, rashes or other
skin abnormalities.
·
Listen to the baby's cry and note its sound.
·
Weigh the baby and plot this reading on its growth chart.
Perform a systematic 'head to toes'
examination
This should be done carefully and in good light to detect abnormalities:
This should be done carefully and in good light to detect abnormalities:
·
Head:
·
Shape, presence of fontanelle and
whether normal, sunken or bulging.
·
Measure and record head circumference
on the growth chart.
·
Assess facial appearance and eye
position.
·
Look for any asymmetry or abnormality
of facial form.
·
Eyes:
·
Establish that they are of normal
shape and appearance.
·
Check for presence of red reflex.
·
Look for obvious cataracts or signs
of ophthalmic infection.
·
Ears:
·
Note shape and size.
·
Establish whether they are set at the
normal level or 'low-set'.
·
Check patency of external auditory
meatus.
·
Mouth:
·
Check the colour of the mucous
membrane; observe the palate.
·
Check suckling reflex by inserting
a clean little finger gently inside the baby's mouth.
·
Arms and hands:
·
Establish whether they are of normal
shape and moving normally.
·
Look for evidence of traction birth
injury (eg, Erb's palsy) by checking the neck, shoulders and clavicles.
·
Count the fingers and observe their
shape; check for any evidence of clinodactyly (incurving of fingers).
·
Check palmar creases - whether they
are multiple or single. A single palmar crease may be normal, but can be a sign
of Down's
syndrome (trisomy 21).
·
Peripheral pulses:
·
Check brachial, radial and femoral
pulses for rate, rhythm and volume.
·
A weak pulse may occur with a
congenital cardiac anomaly (impairing cardiac output and in conjunction with
other signs from the examination).
·
Heart:
·
Check the cardiac position by
palpation and feel for any thrill or heave.
·
Suspected abnormalities require further
examination (and often more expert opinion and investigation).
·
Lungs:
·
Watch the respiratory pattern, rate
and depth for a few seconds.
·
Look for any evidence of intercostal
recession.
·
Auscultate lung fields for added
sounds.
·
Abdomen:
·
Look at abdominal girth and shape.
·
Carefully check the umbilical stump
for infection or surrounding hernia.
·
Palpate gently for organs, masses or
herniae.
·
It is common to be able to feel the
liver and/or spleen in healthy newborns.
·
Palpate for testes in boys.
·
Inspect the anus (establish whether
meconium been passed).
·
Back:
·
Look carefully at the skin over the
back and at the spinal curvature/symmetry.
·
Observe whether there is any evidence
of spina bifida occulta or pilonidal sinus
hidden by flesh creases or dimples.
·
Palpate the spine gently.
·
Hips:
·
Specifically test for congenital
dislocation of the hip (aka congenital
hip dysplasia) using a combination of Barlow and
Ortolani manoeuvres (follow the link for more detail).
·
Legs:
·
Watch movements at each joint.
·
Check for any evidence of talipes
equinovarus.
·
Count toes and check their shape.
·
CNS:
·
Observe tone, behaviour, movements
and posture.
·
Elicit newborn reflexes only if there
is cause for concern.
·
Further examination should be
conducted as necessary according to any abnormalities that are detected, or
suspicions of undetected illness in the baby.
Record findings
·
Always document the findings of the
examination in the postnatal care plan and personal child health record. A
proforma for the examination, kept within the notes, can save time and act as a
prompt to ensure that no element of the examination is missed.
Other screening tests
·
NICE recommends that newborn screening tests, as currently advised by the UK National Screening
Committee, should be offered to all UK-born children.
·
This includes the newborn heel prick
blood spot test traditionally used to pick upphenylketonuria and hypothyroidism. The blood spot, or heel prick, test is taken between 5
and 8 days ((ideally at day 5, count date of birth as day 0). It may include
tests for other metabolic abnormalities in future:
·
Sickle cell
disease: all newborn babies in England are
offered screening for sickle cell disease.[1]
·
Medium-chain acyl CoA dehydrogenase
(MCAD): the Department of Health introduced
routine MCAD deficiency screening in the neonatal blood spot test from February
2009.[1]
·
The routine neonatal screening
examination should be repeated at 6-8 weeks and an assessment of visual
fixation and social smiling conducted.
·
A hearing
screen should be completed before
discharge from hospital (or by week 4 in the hospital programme/week 5 in the
community programme).
Common abnormalities detected in the newborn screening
examination
·
Capillary or macular haemangioma:
·
Also known as stork mark/bites, or
salmon patch; found around the eyes and nape of the neck in 30-50% of babies.
·
Those around eyes normally disappear
in first year, and commonly persist if on the nape of the neck.
·
Blue-black pigmented area:
·
Also known as Mongolian blue spots;
they are seen at the base of the back and on the buttocks. These are common in
dark-skinned parents but can occur in Caucasian infants.
·
They normally disappear over the
first year.
·
Urticaria of the newborn:
·
This is most evident on day 2 as a
fluctuating, widespread erythematous rash with a raised white/cream dot at the
centre of a red flare, mostly apparent on the trunk.
·
This disappears spontaneously without
treatment.
·
Heat rash:
·
Also known as miliaria, this may
appear as either red, macular patches or superficial, clear vesicles that are
most marked on the forehead and around the neck.
·
It is associated with warm humid
environments and will clear in cooler, drier conditions.
·
Breast enlargement:
·
Seen in both girls and boys; the
child may secrete a small amount of milk ('witches milk').
·
This is thought to be due to response
to circulating maternal hormones.
·
It is not significant unless the
condition persists/progresses.
·
White pimples:
·
Also known as milia; they are seen on
the nose and cheeks and are found in approximately 40% of newborns, due to
blocked sebaceous glands.
·
These clear spontaneously.
·
Oral cysts:
·
These are found on the palate near
the midline and on the gums (also known as Epstein's pearls).
·
They may be larger and on the floor
of the mouth.
·
They usually resolve spontaneously.
·
Teeth can be present at birth (no
action is required unless they are loose or abnormal, in which case they may
have to be extracted).
·
Accessory skin tags:
·
Seen on the face as accessory
auricles anterior to the ears.
·
They can be dealt with easily by a
surgical team.
·
Vestigial extra digits:
·
These can usually be dealt with
easily by a surgical team.
·
Sacral dimples:
·
These are common.
·
Examine carefully to detect
underlying sinus or evidence of spina bifida occulta.
·
Deformity of the shape of the head in
the immediate postnatal period and following days is common:
·
Such 'moulding' is non-pathological
and usually resolves spontaneously.
·
Assess whether there are any other
craniofacial abnormalities.
·
Seek expert input if unsure.
Abnormalities that may indicate a significant underlying
cause
·
Any wide separation of the
fontanelles, with presence of islands of bone (Wormian bones) may indicate
cranial abnormalities caused by a range of congenital syndromes.
·
A third fontanelle, found between the
normal anterior and posterior fontanelles may indicate Down's
syndrome.
·
Abnormally shaped or placed ears may
indicate fetal alcohol
syndrome, craniofacial abnormalities due to
abnormal branchial arch development or conditions such asEdward's
syndrome (trisomy 18) or
congenital renal anomalies.
·
Single palmar crease can indicate
Down's syndrome but may be found in children who do not suffer from this
condition.
·
Abnormalities of the face, jaw and
ears are often associated with hearing dysfunction; hearing tests should be
performed and ENT assessment requested.
149. Secondary
powerless labor.
Secondary uterine inertia occurs after adequate uterine
contractions and manifests by decreasing of uterine contractions strength,
duration and frequency later.
Secondary uterine inertia as a rule is presented in the end
of the cervical stage of labor and in the pelvic stage. Its frequency is 2,4 %
to all number of labor. The causes of secondary uterine inertia are the same,
as primary uterine inertia has had. But, as a rule, secondary uterine inertia
is more common as a result of:
·
“cephalopelvic disproportion” in clinic contracted pelvis,
hydrocephalia, fetal malpresentations, transversus and oblique fetal lies,
tumors in true pelvis;
·
“unripe” uterine cervix, its scar’s changes;
·
vaginal stenosis;
· breech presentation;
·
expressed pain in uterine contractions;
·
inadequate usage of amniotomy;
·
endometritis;
·
administration of excess and inadequate anesthesia, uterotonic and
spasmolytics drugs.
It is very important to differentiate secondary uterine
inertia with “cephalopelvic” disproportion for preventing obstetric
complications. Arrest of descent over a 2 hour-period is suggestive of either
“cephalopelvic” disproportion or ineffective uterine contractions.
150. Clinic and
management of premature delivery.
Preterm labour (PTL)
DEFINITION Onset of labour prior to 37 weeks’ gestation.
AETIOLOGY May be idiopathic. Infection (often subclinical)
contributes increasingly to
aetiology with decreasing gestation. See
pathology/pathogenesis below.
ASSOCIATIONS/RISK FACTORS Infection of genital tract, UTI,
multiple pregnancy,
polyhydramnios, cervical incompetence/previous cervical
surgery, systemic infective illness,
previous PTL, uterine abnormalities, APH.
EPIDEMIOLOGY Affects 6% of deliveries in the UK.
HISTORY Regular painful contractions, although may present
as diffuse pains or cramping. ?
PV bleed, ?SROM.
EXAMINATION
General: Signs of infection (tachycardia, fever).
Abdomen: Contractions, abdominal tenderness (may indicate
abruption, chorioamnionitis).
Speculum: ?liquor pooling.
Vaginal: Assess cervical dilatation.
PATHOLOGY/PATHOGENESIS Relationship with systemic infective
illness may be due to
direct spread of infection or release of inflammatory
mediators. Decidual haemorrhage is
also associated with release of inflammatory mediators. PTL
in polyhydramnios/multiple
pregnancy is related to uterine over-distension.
INVESTIGATIONS
Bloods: FBC, CRP (evidence of infection).
Microbiology: MSU, LVS/HVS.
CTG: Fetal wellbeing.
USS: Confirm presentation, estimated fetal weight, cervical
length.
Fetal fibronectin: (Sample taken at speculum examination)
predictive of likelihood to labour
(used for high negative predictive value).
MANAGEMENT Administer steroids (fetal lung maturation),
tocolysis used to allow steroid
cover e.g. oxytocin antagonists (atosiban), nifedipine or
GTN (contraindicated if APH or
evidence of infection), antibiotics if SROM, fetal monitoring.
COMPLICATIONS Respiratory distress syndrome, intracranial
haemorrhage, sepsis,
necrotising enterocolitis, neurodevelopmental delay,
cerebral palsy, neonatal death.
PROGNOSIS Dependent on gestation at delivery, accounts for
over 20% of perinatal
mortality. There is 20% risk of PTL in subsequent
pregnancies
151. Dyscoordinated
labor activity.
INCOORDINATIVE UTERINE ACTIVITY is characterized by absence
adequate coordinate uterine contractions
between different uterine parts: right and left its sides, upper and lower
uterine parts, different its regions. Its frequency is 1-3 %.
The main causes of incoordinate uterine contractions are:
· uterine abnormality;
· uterine cervix dystocia;
· flat amniotic sac;
· impairment of uterine innervating;
· damaging of uterine regions as a result of inflammatory,
degenerative and neoplastic changes.
Incoordinative uterine activity is characterized by painful,
irregular and frequent uterine
contractions. “Unripe” cervix and slow its dilation, preterm rupture of
amniotic fluid, flat amniotic sac is common. Presenting part is movable or
fixated to the pelvic inlet for a long period of time. Woman in labor has been tired later and the arrest of
presenting part is presented..
It is very important to differentiate incoordinative uterine
activity with uterine inertia, “cephalopelvic” disproportion for different ways
of these disorders management.
Management of abnormal labor in incoordinative uterine
activity
Amniotomy gives good results. Uterotonic drugs are contraindicated
for incoordinative uterine activity treatment.
The b-mimetics, calcium chammel blockers, magnesium sulfate,
and antiprostaglandins and sedative drugs have been used to inhibit labor.
Ethanol has a direct depressant effect on smooth muscle and inhibits oxytocin
release. Atropine and scopolamine relax the lower uterine segment and decrease
the frequency of contractions
In the case of presence maternal exhaustion, therapeutic
rest is indicated. In the case of “cephalopelvic” disproportion cesarean section
is performed.
152. Amniotomy
(indications, technique).
indications
Induction or augmentation of labour, with or without a
Syntocinon infusion
ƒ To apply a scalp electrode for foetal
monitoring
ƒ To take foetal lactate/pH
ƒ Assessment of liquor in presence of
abnormal foetal heart rate
ƒ Non-reassuring CTG
technique
153. Hydatidiform mole.
Clinic, diagnostic, management.
Gestational
trophoblastic disease (hydatidiform mole)
DEFINITION
Benign
tumour of trophoblastic tissue.
AETIOLOGY
Abnormal
fertilisation.
Complete
moles: Diploid and paternal in origin with no fetal tissue, usually arise
from
duplication
of haploid sperm after fertilisation of an empty ovum, or from dispermic
fertilisation
of an empty ovum.
Partial
moles: Triploid with two sets of paternal haploid genes and one set of
maternal
haploid
genes following dispermic fertilisation of an ovum, may contain fetal parts or
fetal
red
blood cells.
ASSOCIATIONS/RISK
FACTORS See Gestational trophoblastic malignancy.
EPIDEMIOLOGY
Affects
1 per 1500 pregnancies (up to 1 per 200 in parts of Asia).
HISTORY
PV
bleeding, hyperemesis (" bHCG), symptoms of hyperthyroidism are rare (note:
often
diagnosed by USS prior to symptoms).
CLINIC:
Uterus
larger than expected for gestational age, rarely signs of
hyperthyroidism
("bHCG
mimics TSH).
PATHOLOGY/PATHOGENESIS
Macro:
Grape-like appearance in complete moles, partial moles may contain recognisable
fetal
tissue.
Micro:
Hydropic villi, atypical hyperplastic trophoblasts in complete moles, focal
villi swelling
and
trophoblastic hyperplasia in partial moles.
INVESTIGATIONS
Bloods: bHCG
grossly elevated.
Imaging:
Pelvic USS (snowstorm appearance, vesicles/cysts).
MANAGEMENT
Surgical:
ERPC (avoid uterotonics owing to possibility of dissemination).
Monitoring:
Serial monitoring of bHCG in specialist centre (in UK: Charing Cross, Sheffield,
Dundee),
methotrexate if rising/stagnant bHCG levels,
avoid pregnancy until 6 months
of
normal bHCG levels.
COMPLICATIONS
Progression
to malignancy (15–20% complete moles, 2–3% partial
moles).
PROGNOSIS
Risk
of recurrence is 1–2%. After two or more molar pregnancies, recurrence
risk
is 17%.
154. Diabetes mellitus
and pregnancy.
Diabetes may complicate a
pregnancy either because
a woman has pre-existing
insulin-dependent diabetes
mellitus (IDDM) or
non-insulin-dependent diabetes
mellitus (NIDDM) before
pregnancy or because she
develops usually transient
impaired glucose tolerance
or diabetes during the
course of her pregnancy(Gestational diabetes).
Effects of pregnancy on diabetes
• Change in eating pattern
• Increase in insulin dose requirements
• Greater importance of tight glucose control
• Increased risk of severe hypoglycaemia
• Risk of deterioration of pre-existing retinopathy
• Risk of deterioration of established nephropathy
Effects of diabetes on pregnancy
• Increased risk of miscarriage
• Risk of congenital malformation
• Risk of macrosomia
• Increased risk of pre-eclampsia
• Increased risk of stillbirth
• Increased risk of infection
• Increased operative delivery rate
Management in
pregnancy
The aim of treatment is to maintain blood glucose
levels as near normal as possible with a combination of
diet and insulin, and control is achieved by matching
insulin dose with food intake. Insulin doses will change
in pregnancy due to the physiological increase in
insulin resistance. Targets for therapy are to maintain
HbA1c
6 per cent, with pre-meal glucose levels at
3.5–5.5 mmol/L and 2-hour postprandial levels of
4–6.5 mmol/L. This relative normoglycaemia means
that asymptomatic hypoglycaemia can occur more
frequently. Therefore, careful self-monitoring of glucose
levels by women with diabetes is a critical aspect of care.
Obstetric management is aimed initially at
ensuring that the appropriate screening tests are
performed, including nuchal translucency scanning,
detailed ultrasound assessment for fetal anomalies
and fetal echocardiography. Serial growth scans
are recommended to detect fetal macrosomia and
polyhydramnios, although this is rarely a problem
before the third trimester. Any concern for fetal
well-being should lead to increased surveillance
with Doppler ultrasound and CTG.
155. Placenta previa:
etiology, diagnostic, management.
Placenta
praevia
DEFINITION
A
placenta wholly or partly inserting into the lower segment.
AETIOLOGY
Unknown.
ASSOCIATIONS/RISK
FACTORS Multiple pregnancy, "maternal age, previous uterine
surgery,
previous placenta praevia, smoking.
EPIDEMIOLOGY
Affects
0.5% of pregnancies.
HISTORY
Usually
detected on routine ultrasound. Acutely, presents with painless PV
bleeding
in the second/third trimester.
EXAMINATION
General: May
be shocked (tachycardia, hypotension)
Abdomen:
Usually soft, non-tender, ?malpresentation.
Vaginal:
Contraindicated.
Speculum:
Gentle speculum examination permitted (assess bleeding).
PATHOLOGY/PATHOGENESIS
Unknown.
Abnormal implantation thought to occur where
blood
supply disrupted (e.g. uterine scar). Bleeding occurs secondary to shearing
forces.
Minor
placenta praevia: Placenta close to, but not covering, internal cervical os.
Major
placenta praevia: Placenta overlying internal cervical os.
INVESTIGATIONS
Bloods:
FBC, U&E, clotting, X-match.
CTG:
Fetal wellbeing.
USS:
Confirm placental site.
MRI: May
be used to determine placenta accreta (placenta adherent to uterine wall) or
placenta
increta/percreta (placenta invades into or through the uterine wall).
MANAGEMENT
Vaginal
delivery: Only possible if lower placental edge >2 cm
from internal os and fetal
head
below placenta.
Caesarean
section: If no bleeding, aim for 38/40.
Mild
self-limiting bleed, no fetal/maternal compromise: Admit for
monitoring, steroids
if
preterm, anti-D if Rhesus negative.
Severe
bleed or fetal/maternal compromise: Manage as for severe placental
abruption or
maternal/fetal
compromise (see above).
COMPLICATIONS
Maternal:
Haemorrhage (APH þ " risk PPH), DIC, hysterectomy.
Fetal:
IUGR, death.
PROGNOSIS
Maternal
mortality is 1 per 300.
156. Pregnancy and
labor after surgical treatment of heart diseases.
157. Perinatal centers.
Perinatal Center is a
hospital that provides care services for pregnant women and newborns . There
are 3 levels
Level 1: hospitals
provide care to normal and low-risk pregnant women and newborns, and they do
not operate neonatal intensive care units (NICU);
Level 2 : hospitals
provide care to women and newborns at moderate risk and do operate NICUs;
Level 3 : hospitals
care for patients requiring increasingly complex care and operate NICUs.
158. Amniotic fluid.
Indications for amniotomy.
Amnionic fluid serves several roles during pregnancy. It
creates
a physical space for fetal movement, which is necessary
for normal musculoskeletal development. It permits fetal
swallowing—essential for gastrointestinal tract development,
and fetal breathing—necessary for lung development. Amnionic
fluid guards against umbilical cord compression and protects
the fetus from trauma. It even has bacteriostatic
properties.
Amnionic fluid volume abnormalities may reflect a problem
with fluid production or its circulation, such as underlying
fetal
or placental pathology. These volume extremes may be
associated
with increased risks for adverse pregnancy outcome.
159. Artificial
induction of labor. Indications for induction of labor.
Indications
Induction is indicated when the benefits to either mother or
fetus outweigh those of pregnancy continuation. The more
common indications include membrane rupture without labor,
gestational hypertension, oligohydramnios, nonreassuring
fetal
status, postterm pregnancy, and various maternal medical
conditions such as chronic hypertension and diabetes.
Technique
Oxytocin has been used for decades to induce or augment
labor. Other effective methods include prostaglandins, such
as misoprostol and dinoprostone, and mechanical methods
that encompass stripping of membranes, artificial rupture of
membranes, extraamnionic saline infusion, transcervical
balloons,
and hygroscopic cervical dilators. Importantly, and as
recommended in Guidelines for Perinatal Care, each
obstetrical
department should have its own written protocols that
describe administration of these methods for labor induction
and augmentation (American Academy of Pediatrics
and American College of Obstetricians and Gynecologists,
2012).
160. Role of female
dispensary in prevention of gestosis.
Head of obstetrics and
gynecology chair L.V. Gutikova

















































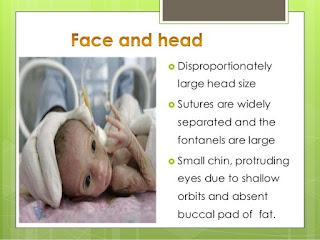





































No comments:
Post a Comment